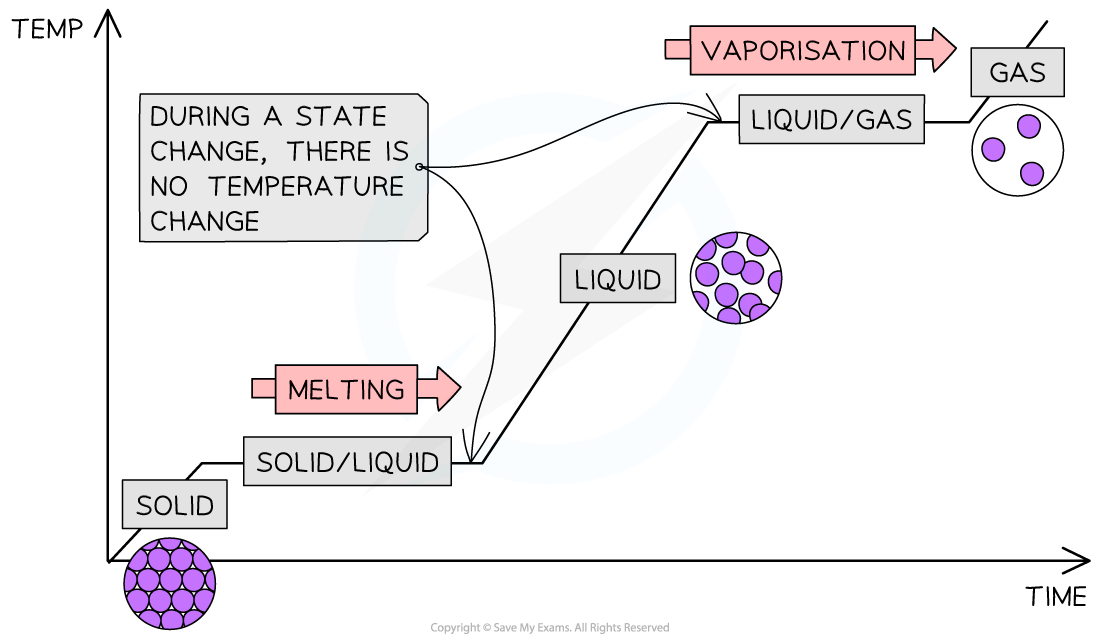
Heating Curve For A Pure Substance . Heating curves show how the temperature changes as a substance is heated up. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. This exercise explores the changes that occur to a. They show how the temperature changes as a substance is. As we heat pure water, the temperature gradually increases until it. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Plateaus in the curve (regions of constant. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves.
from www.linstitute.net
As we heat pure water, the temperature gradually increases until it. This exercise explores the changes that occur to a. Heating and cooling curves are graphs. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. For heating curves, we start with a solid and add heat energy. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Plateaus in the curve (regions of constant. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. Heating curves show how the temperature changes as a substance is heated up. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat.
CIE IGCSE Biology 复习笔记:1.1.2 States of Matter
Heating Curve For A Pure Substance Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. They show how the temperature changes as a substance is. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. As we heat pure water, the temperature gradually increases until it. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. Heating curves show how the temperature changes as a substance is heated up. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. Heating and cooling curves are graphs. This exercise explores the changes that occur to a. Cooling curves are the opposite. Plateaus in the curve (regions of constant. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves.
From askfilo.com
The graph shows the heating curve for a pure substance. The temperature r.. Heating Curve For A Pure Substance Boiling or vaporization is an example of a phase change from the liquid to the gas phase. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. As we heat pure water, the temperature. Heating Curve For A Pure Substance.
From askfilo.com
The graph below shows the heating curve for a pure substance. The tempera.. Heating Curve For A Pure Substance They show how the temperature changes as a substance is. Heating and cooling curves are graphs. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. For heating curves, we start with a solid and add heat energy. The representative heating curve for a substance depicts changes in temperature that. Heating Curve For A Pure Substance.
From www.coursehero.com
[Solved] Given below is the graph of a substance's heating curve Heating Curve For A Pure Substance Heating and cooling curves are graphs. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Plateaus in the curve (regions of constant. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. Cooling curves are. Heating Curve For A Pure Substance.
From scienceisntscary.wordpress.com
Image Heating Curve For A Pure Substance Plateaus in the curve (regions of constant. For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. This exercise explores the changes that occur to a. There are different ways to separate. Heating Curve For A Pure Substance.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve For A Pure Substance They show how the temperature changes as a substance is. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. Heating curves show how the temperature changes as a substance is heated up. For heating curves, we start with a solid and add heat energy. The representative heating curve for a substance depicts changes in. Heating Curve For A Pure Substance.
From askfilo.com
The graph shows the heating curve for a pure substance. The temperature r.. Heating Curve For A Pure Substance The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. Heating and cooling curves are graphs. They show how the temperature changes as a substance is. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Plots of the temperature of. Heating Curve For A Pure Substance.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve For A Pure Substance They show how the temperature changes as a substance is. Heating and cooling curves are graphs. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. Heating curves show how the temperature changes as a substance is heated up. To identify the purity of a substance, we can use heating curves, such as. Heating Curve For A Pure Substance.
From www.doubtnut.com
The temperaturetime graph below shows the heating curve for pure wax Heating Curve For A Pure Substance Boiling or vaporization is an example of a phase change from the liquid to the gas phase. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. They show how the temperature changes as a substance is. This exercise explores the changes that occur to a. As we heat. Heating Curve For A Pure Substance.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Heating Curve For A Pure Substance Heating and cooling curves are graphs. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. For heating curves, we start with a solid and add heat energy. As we heat pure water, the temperature gradually increases until it. Plateaus in the curve (regions of constant.. Heating Curve For A Pure Substance.
From www.numerade.com
SOLVED The following graph represents the heating curve of a Heating Curve For A Pure Substance Heating curves show how the temperature changes as a substance is heated up. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Plateaus in the curve (regions of constant. Cooling curves are the opposite. Boiling or vaporization is an example of a phase change from. Heating Curve For A Pure Substance.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.2 2 Pure Substance vs Mixture翰林国际教育 Heating Curve For A Pure Substance As we heat pure water, the temperature gradually increases until it. Heating curves show how the temperature changes as a substance is heated up. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. This exercise explores the changes that occur to a. Cooling curves are the opposite. There. Heating Curve For A Pure Substance.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Heating Curve For A Pure Substance As we heat pure water, the temperature gradually increases until it. They show how the temperature changes as a substance is. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of. Heating Curve For A Pure Substance.
From www.youtube.com
Comparison between heating and cooling curves of pure and impure Heating Curve For A Pure Substance Heating curves show how the temperature changes as a substance is heated up. As we heat pure water, the temperature gradually increases until it. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating and cooling curves are graphs. To identify the purity of a. Heating Curve For A Pure Substance.
From brainly.in
Q2. The given graph shows the heating curve for a pure substance,the Heating Curve For A Pure Substance This exercise explores the changes that occur to a. Cooling curves are the opposite. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. They show how the temperature changes as a substance is. Plateaus in the curve (regions of constant. To identify the purity of. Heating Curve For A Pure Substance.
From www.doubtnut.com
The temperaturetime graph below shows the heating curve for pure wax Heating Curve For A Pure Substance For heating curves, we start with a solid and add heat energy. Plateaus in the curve (regions of constant. To identify the purity of a substance, we can use heating curves, such as the graph below for pure water. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. There are different ways. Heating Curve For A Pure Substance.
From www.owhentheyanks.com
Heating And Cooling Curve Worksheet Heating Curve For A Pure Substance Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. As we heat pure water, the temperature gradually increases until it. Boiling or vaporization is an example of a phase change from the liquid to the gas phase. They show how the temperature changes as a. Heating Curve For A Pure Substance.
From worksheetlistmo.z21.web.core.windows.net
Heating Curve Of Water Worksheets Answers Heating Curve For A Pure Substance Heating curves show how the temperature changes as a substance is heated up. As we heat pure water, the temperature gradually increases until it. Plateaus in the curve (regions of constant. The representative heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. Plots of the temperature of a substance versus. Heating Curve For A Pure Substance.
From preparatorychemistry.com
Heating Curve Heating Curve For A Pure Substance Heating curves show how the temperature changes as a substance is heated up. There are different ways to separate mixtures, for example by filtration, crystallisation, distillation, or chromatography. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. This exercise explores the changes that occur to a.. Heating Curve For A Pure Substance.